Determination of
the maximum shift of lambda max of a dye, due to a red shift (or
bathochromic effect)*.
Trypsin a protease of 25,5 KD is known to bind the thionin (or lauth's violet) in its enzymic active site ( GLAZER, 1967). The spectrum of the trypsin bound thionin shows a red shift compared to the spectrum of the free thionin.
To determine the wavelength showing the maximum absorbance difference, it is necessary to perform a difference spectrum (spectrum of free dye subtracted from the spectrum of the bound dye).
This experiment shows the advantage of using the baseline function to make a difference spectrum (spectrometer; Spectronic 301).
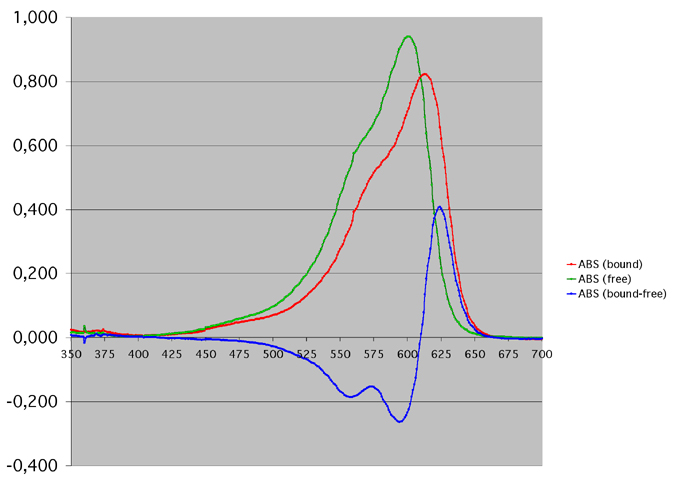
- Indeed, we can measure a baseline with a cuvette containing only the buffer, then measure the spectrum of the free thionin, then measure the spectrum of the trypsin bound thionin, and then finally subtract the two spectrums. We get the following graphs: The difference spectrum in blue shows a maximum positive peak at 624 nm.

Difference spectrum (blue) of free thionin (green) and trypsin bound thionin (red). The bound thionin is in a solution containing excess trypsin (17 µM thionin, 170 µM trypsin)
- Another straight way to obtain the difference spectrum is to make the baseline with the free thionin solution and then make the spectrum of the thionin bound trypsin using this baseline. Thus we get with Datalyse the difference spectrum.
The next picture shows the baseline measured with a cuvette containing the free thionin:
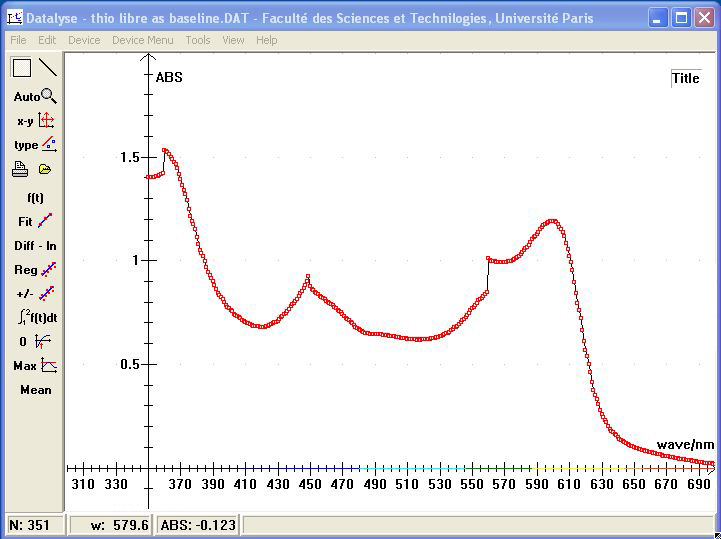
The following picture shows the difference spectrum. The "Max" function of Datalyse found a maximum at 623,95 nm:

The next picture shows a graph containing the difference spectrums obtained by the two different methods. The results are very similar.
Limitations of the device:
* Official definition of bathochromic shift (effect):
Shift of a spectral band to lower frequencies (longer wavelengths) owing to the influence of substitution or a change in environment. It is informally referred to as a red shift and is opposite to hypsochromic shift (blue shift). 1994, 66, 1088; 1996, 68 , 2230 .IUPAC Compendium of Chemical Terminology 2nd Edition (1997)
Bibliography:
GLAZER A.N. Journal of Biochemistry (JBC) 1967 Vol 242 n° 14 3326 - 3331
Products:
- Thionin (Lauth's violet, thiazin class of dyes, final product of the oxidative demethylation of methylene blue) Sigma T3387 (C.I. 52000) (Merck Index 12 , 9483 ·Beilstein 27 , 392 IV , 5149 ·BRN: 746016 ·Conn's IX , 417 , 602 ·)
- Trypsin from porcine pancreas, Sigma T0303
Datalyse web site: http://www.datalyse.dk/datauk/
Address: Gilles Carpentier, Faculté des Sciences et Technologies, Université Paris 12 Val-de-Marne, France.
Gilles Carpentier's Web Site: "Computer Data Acquisition for Biochemistry Practice Works: Methods and Examples"