This page describes an easy way to check a spectrometer by using a solution of potassium dichromate (K2Cr2O7). This compound is known to show in solution two particular peaks of absorbance, one at 257 nm (UV) and one at 350 nm (visible - near UV). The absorbance values at these wavelengths are usually used to calibrate the OD values of spectrometers, by making very precisely the solution with dried powder (S. Ebel, 1992). Here, we just consider the lambda checking. A table at the end of this document is given to make OD checking solution.
A rapid solution, giving an appropriate OD to check the wavelength of a spectrometer, can be made as follows :
- Concentrated solution (A): 10 mg of K2Cr2O7 in 10 ml of H2O.
- Solution ready to use: 10 ml of solution A diluted to 1/20, added to one drop of Pasteur pipette of H2SO4 1M.

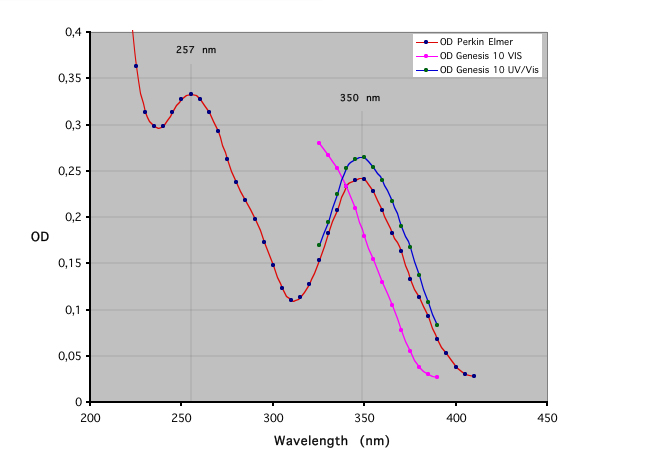
- The large spectrum (200 to 500 nm), made with a double beam Perking Elmer spectrometer give one peak at 257.1 nm and one peak at 349.9 nm. It can be considered as a reference spectrum.
- The two other spectrums, (325 to 380 nm) are made with a Genesis 10 UV/visible used in visible and a Genesis 10 visible, both simple beam spectrometers (manually collected data). Note the correct 350 nm peak for the Genesis 10 UV/Vis, and the wrong spectrum for the genesis 10 visible model. If you obtain such spectra, contact your retailer to make calibrate your device !
For Genesis family, see the support at Thermo Electron Corporation (www.thermo.com/com/cda/servicesupport/home/) or contact VWR International.
References
:
Absorbance of a solution of K2Cr2O7 at 60,06 mg/l in 5mM H2SO4:
257 nm; 0,868
350 nm; 0,640
S. Ebel, Fresenius. J. Anal. Chem. 1992, 324, 769
Gilles Carpentier's Web Site: "Computer Data Acquisition for Biochemistry Practice Works: Methods and Examples"