
Gilles Carpentier's Web Site:
This page describes
an easy and cheap method to make numerical data acquisition from a
palarographic oxymeter signal. Leland C. Clark described in 1962 a
polarographic device the " Clark electrode ", allowing to measure the
glucose in blood, by following the decrease of dissolved
O2, due to the glucose oxydase enzyme activity.
L.C.
Clark is
generally considered as the inventor of the first biosensor.
The
principle of the method:
Briefly, the probe consists in an anode (Pt) and a cathode (Ag) kept in contact with a thin bridge of paper, soaked with a saturated solution of KCl. This electrolytic bridge and the anode are enclosed below a membrane (Teflon or polypropylene) permeable to O2 but not to irons or organic compounds. This membrane is polarized by a control device (about 0.6 V). O2 is soluble in water, and by diffusion through the membrane, can react with the Ag of the cathode. The current of this oxydo-reduction (at the constant polarizing voltage of 0.6 V) is directly proportional to the partial pressure of oxygen diffusing to the reactive surface of the electrode. The controle module converts this current into a tension which allows to measure the variations of O2 concentrations. More details about the physico-chemical principle are avaible on the "Experimental Biosciences" web site.
The device with the acquisition montage: the Metex M-3850D RS-232
port is connected to a USB port computer using a RS232 to USB adaptor
(see How
to use Datalyse on a USB computer, How
to use Datalyse on a USB Apple Macintosh notebook, for computer compatibilities, and Metex
family description on Datalyse website).
The detailed mounting preocedure of the
probe is descibed step by step in the page "Clark
probe - pratical note".

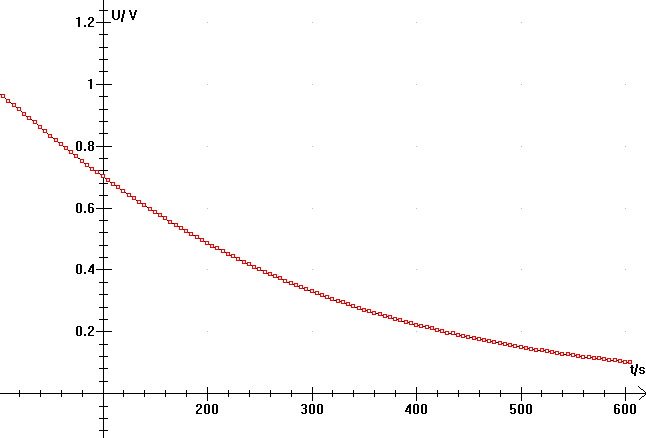
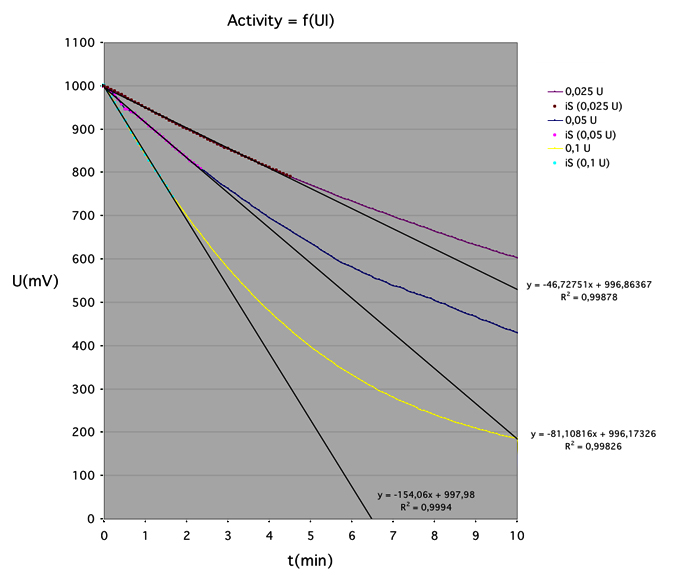
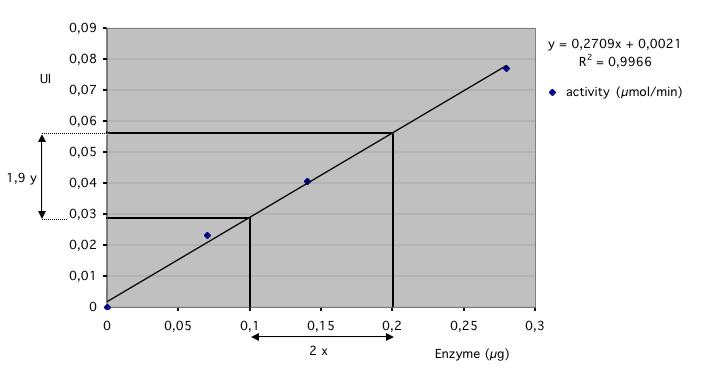
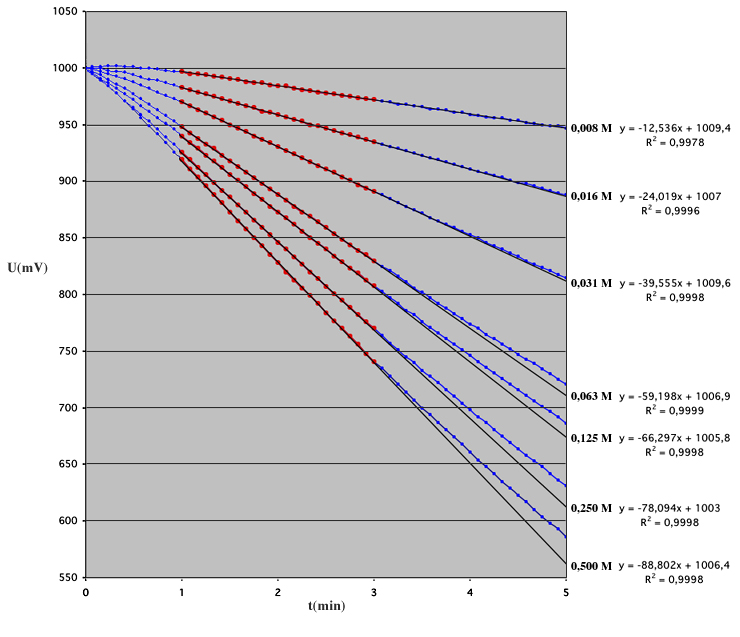
Here is an example of the acquisition of an Glucose Oxydase (GO) activity: The kinetic was followed during 10 minuts with an acquisition every 5 seconds. Note the initial linear speed of the catalyse, following by a nonlinear step due to the decrease of the O2 concentration. The concentration of the dissolved oxygen in water, is a limit of the method. (contents of the cell; 2 ml of 0.5 M of beta-D-glucose, with about 0.05 UI of GO, pH 5 in 50 mM phosphate buffer, 25°C). In these conditions, the glucose concentration is in large excess comparing to the Michaelis-Menten constant.

Products:
Glucose Oxydase from aspegillus niger from Sigma, G.9010.
beta-D-Glucose from Prolabo, 24379.294.
Special thanks to Alessandra Albano for her participation to
the English correction of this page.
Datalyse web site: http://www.datalyse.dk/datauk/
Address: Gilles Carpentier, Faculté des Sciences et Technologies, Université Paris 12 Val-de-Marne, France.
Gilles Carpentier's Web Site: "Computer Data Acquisition for Biochemistry Practice Works: Methods and Examples"